10–12 November 2025, Glasgow, United Kingdom
The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) is one of the world’s most influential professional organisations in health economics and health technology assessment (HTA). It brings together experts from academia, research institutions, regulatory bodies, the healthcare industry and international organisations. ISPOR is committed to advancing evidence-informed decision-making, improving healthcare value, promoting cross-national knowledge exchange, and driving methodological development in HTA, pharmacoeconomics, real-world evidence generation and value-based healthcare systems.
As one of the largest global conferences in health economics and HEOR, ISPOR Europe attracts thousands of participants each year to discuss the latest developments in HTA methodologies, payer decision-making, pharmaceutical pricing reforms, and real-world data and evidence generation practices.
The 2025 ISPOR Europe conference was successfully held in November in Glasgow, United Kingdom. Centred on the theme “Powering Value and Access Through Patient-Centred Collaboration”, the conference explored how health systems can achieve scientific, transparent and equitable decision-making in the context of increasing system pressures, regulatory change and rapid technological innovation. Multiple ECHORA members and Chinese scholars actively participated in the conference, presenting frontier research across health equity, pharmaceutical pricing, EU HTA reform, chronic disease management and methodological innovation. Their excellent contributions demonstrated the growing presence and impact of Chinese scholars in the global HEOR community.
ECHORA Pre-Conference Gathering
Ahead of the conference, ECHORA organised an in-person gathering for members and Chinese scholars from the UK, continental Europe, China and North America. Warm lighting, relaxed conversations and familiar academic topics created an atmosphere that felt both intellectually stimulating and deeply personal. The event served not only as a platform for professional exchange, but also as a meaningful reunion among friends.
Discussions spanned research collaboration, career development, PhD training, industry opportunities and trends in academic exchange. In addition to a dinner in central Glasgow, attendees also joined a city walk, strolling along the River Clyde, passing through the historic clock-tower district, and visiting the iconic campus of the University of Glasgow.
The gathering concluded with group photos and lively conversations, planting new seeds for future collaboration and community building.


ECHORA Members and Chinese Scholars at ISPOR Europe 2025



Forum: Making Health Equity Actionable in HTA: From Frameworks to Implementation
Associate Professor Xiaoning He presented “Health Inequality Aversion in China: Public and Decision-Maker Views”, offering a rigorous comparison of Chinese public and policymaker preferences regarding health resource allocation. The findings provide valuable reference points for health resource allocation and value-based decision-making in China, contributing important insights to global discussions on incorporating equity within health economic evaluations.


Forum: Drug Pricing in Theory and in Practice: Value in Health Showcase
Chaired by Value in Health Editors-in-Chief C. Daniel Mullins and Nancy J. Devlin, this forum examined the theory and international experience of pharmaceutical pricing reforms. Associate Professor Jiang’s contributions included theoretical evaluations of indication-specific value-based pricing (ISVBP), policy assessments of international reference pricing (IRP) in Europe, and cost-effectiveness evidence supporting CMS drug negotiations (IPAY 2027) in the United States.
The research indicates that while ISVBP aims to align prices more closely with treatment value, its impact on patient welfare may be mixed, particularly when out-of-pocket costs remain high. Experience from European IRP shows that price reductions are not always sustainable and may lead to delayed or foregone market entry. The forum highlighted the crucial role of evidence-based comparative analysis in designing pharmaceutical pricing policies.
Associate Professor Jiang’s study, “Patient Welfare Implications of Indication-Specific Value-Based Pricing of Multi-Indication Drugs”, received the Value in Health Paper of the Year Award.
Poster Tour Presentation: Comparative Effectiveness of Second-Line Antidiabetic Medications on the Risk of Major Adverse Cardiovascular Events: A Real-World Study in China
Using real-world electronic health records from China, this study applied survival models to compare the risk of major adverse cardiovascular events (MACE) across commonly used second-line antidiabetic therapies. Both intention-to-treat (ITT) and per-protocol analyses were conducted to capture treatment effects accurately.
Findings showed that SGLT-2 inhibitors, DPP-4 inhibitors, GLP-1 receptor agonists and thiazolidinediones were associated with lower MACE risks compared with insulin, whereas α-glucosidase inhibitors showed relatively higher risk. This work contributes robust real-world evidence for personalised diabetes treatment and long-term disease management.
The poster was recognised as a Top 5% Finalist and awarded Best Student Poster Research Presentation.
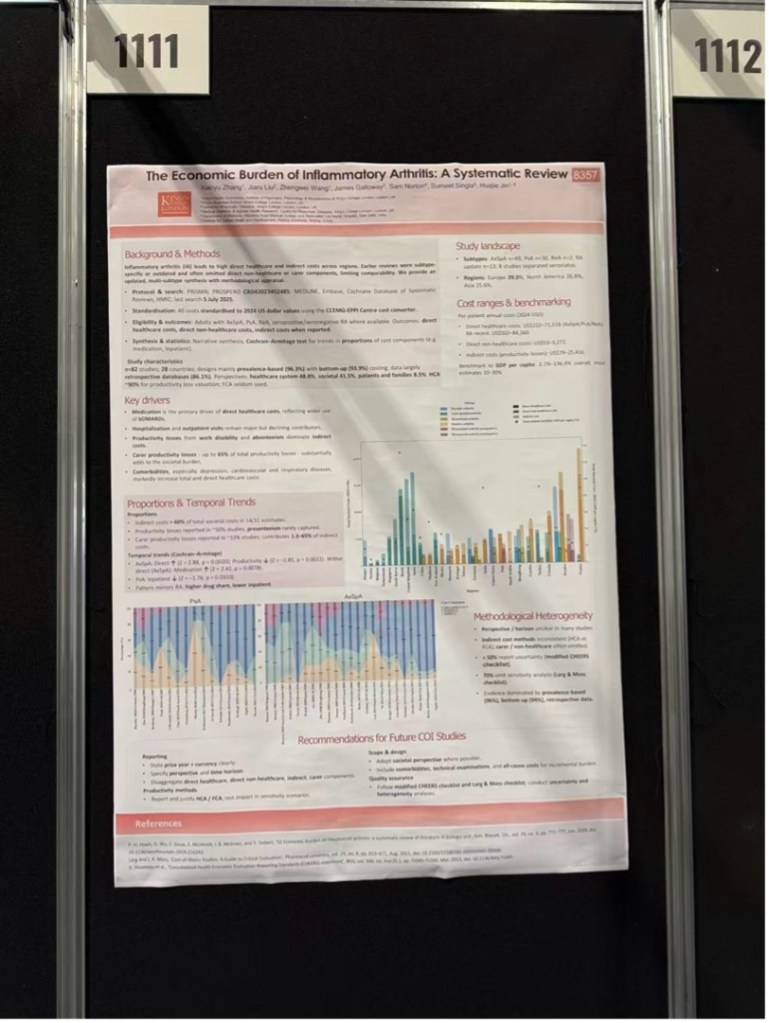
Poster: The Economic Burden of Inflammatory Arthritis: A Systematic Review
This systematic review included 79 global cost-of-illness studies and revealed substantial variation in the annual economic burden of inflammatory arthritis, ranging from approximately USD 1,000 to over USD 80,000 after cost standardisation. Rheumatoid arthritis (RA) and psoriatic arthritis (PsA) were associated with the highest burden, and direct healthcare costs have grown to comprise the largest share in recent years.
The review highlighted several gaps in existing cost studies, including limited sensitivity analyses and insufficient consideration of informal care costs, underscoring the need for more comprehensive and methodologically rigorous future research.
The University of Manchester – Researcher Sainan Chang
Poster: Economic Evaluation of Synovial Biopsy-Guided Treatment Strategy After TNF-alpha Inhibitor Failure in Rheumatoid Arthritis
This study evaluated the cost-effectiveness of using ultrasound-guided synovial biopsy testing to inform biological treatment selection following TNF-alpha inhibitor failure in rheumatoid arthritis. Conventional treatment pathways typically rely on “trial-and-error” approaches, which can increase healthcare costs and delay effective treatment initiation.
Recent trial evidence suggests that patients with low B-cell expression identified via synovial biopsy respond better to tocilizumab than rituximab. Using a Markov model, the study compared costs and health outcomes between the biopsy-guided strategy and usual care.
Results indicate that to meet the NICE cost-effectiveness threshold, substantial discounts (over 40%) on biological therapies, alongside reduced testing costs, would be required. As an early health economic evaluation, the study provides initial insights into key parameters influencing cost-effectiveness and offers valuable guidance for future clinical research.
Written by: Kang Wang

